Introducing CoolTone™ in High Point & Greensboro NC
The Latest Body Contouring Innovation From Allergan
As the leader in body contouring and medical aesthetics, we are pleased to announce the 510(k) clearance of the CoolTone™ device, an innovation in magnetic muscle stimulation (MMS) and the newest addition to our body contouring portfolio. See full indication below.
The CoolTone Device
The Benefits of CoolTone™
✓ Strengthens, tones, and firms the muscles of the abdomen, thighs, and buttocks
✓ Adds another option to your body contouring offerings
✓ Helps attract a new dimension of body contouring patients to your practice
HOW COOLTONE WORKS
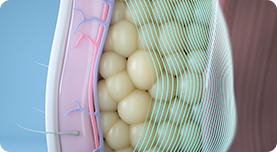
01.
CoolTone’s Magnetic Muscle Stimulation, or MMS technology, penetrates through the skin and fat layers to target only the muscle layer, inducing involuntary muscle contractions.
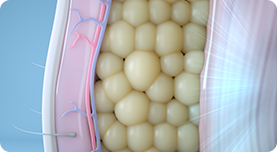
02.
The body’s response to these contractions is to strengthen its muscle fibers, resulting in improved muscle conditioning.
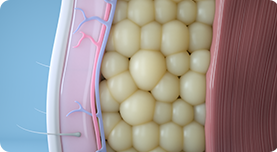
03.
After treatments, abdomen, buttocks, and thighs are firmer and have a more defined and toned appearance.
_________
Indications
The CoolTone™ device is indicated for improvement of abdominal tone, strengthening of the abdominal muscles, and development for firmer abdomen. CoolTone™ is also indicated for strengthening, toning and firming of buttocks and thighs.
Important Safety Information
CoolTone™ should not be used in the head or heart area. CoolTone™ treatment is contraindicated in placing the active applicator over metal or electronic implants/devices in the treatment area like cardiac pacemakers, cochlear implants, intrathecal pumps, implanted defibrillators, implanted neurostimulators, drug pumps, and hearing aids. CoolTone™ is also contraindicated in placing the active applicator over menstruating uterus, over areas of the skin that lack normal sensation, and for patients with fever, malignant tumor, hemorrhagic conditions, epilepsy, recent surgical procedure application in the area of growth plate, pulmonary insufficiency, pregnancy, sensitivity or allergy to latex.
CoolTone™ should be used with caution in patients with Grave’s disease, active bleeding disorders or seizure disorders.
Women who are close to menstruation may find that it comes sooner or cramping is increased or intensified with CoolTone™ treatments, therefore it is recommended to not undergo treatment during this time of the month.
Ensure that persons with pacemakers are not present in vicinity of the device during treatment.
The patient must not be left unattended during treatment.
Adverse effects may include, but are not limited to muscular pain, temporary muscle spasm, temporary joint or tendon pain, and local erythema or skin redness.
Consult the CoolTone™ User Manual for a complete list of Contraindications, Warnings, Precautions, and potential side effects. Treatment applications that deviate from the guidelines are not recommended.
References: 1. Medical Insight, May 2019. 2. Data on file at Allergan.


